Lab Skills & Separating Methods
This unit deals with lab equipment and methods of separating solutions.
Element, Compounds and Mixtures
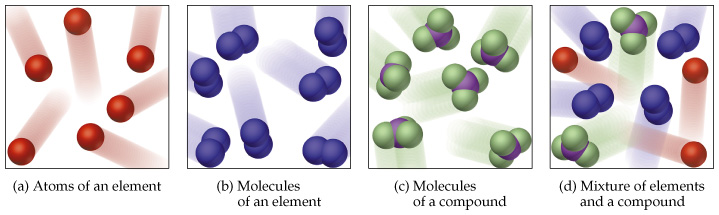
Speaking about the chemistry of matter, we have only 3 types of matter. These are elements, mixtures and compounds. Long ago, scientists found out that the smallest unit of a matter is called an atom. An element is extremely pure because it is made up of only one type of atoms. For example a pure gold ring has only the element Gold (Au) in it. Compounds are very pure too, a compound is made up of one type of a particle called molecule. A molecule consists of two or more atoms chemically bonded together. Carbon Dioxide (CO2) gas is a compound. A mixture however is not pure at all. A mixture is just two or more elements or compounds mixed together, but not chemically bonded. For example if you dissolve some table salt, which is a compound called sodium chloride (NaCl) in some water, which is also another compound (H2O), you will get a mixture of Sodium Chloride in water, but there are absolutely no bonds between the Sodium Chloride molecules and water molecules. Air is another good example of mixtures. Air is just a mixture of gases floating around each other like Nitrogen and Oxygen, which are pure elements. Air also contains compounds such is Carbon Dioxide.
Elements:
Elements are substances that consist of only one kind of atoms and cannot be broken down into simpler substances by chemical means.
Can you recognize elements, compounds and mixtures?
Since particles in mixtures have no chemical bonding between them, they could be easily separated by physical means. The method of separation however depends on the type of the mixture, and some of the physical properties of its components.
We have 4 types of mixtures:
Elements are substances that consist of only one kind of atoms and cannot be broken down into simpler substances by chemical means.
Can you recognize elements, compounds and mixtures?
- An element contains just one type of atom.
- A compound contains two or more types of atom joined together.
- A mixture contains two or more different substances that are not joined together.
- The different substances in a mixture can be elements or compounds.
Since particles in mixtures have no chemical bonding between them, they could be easily separated by physical means. The method of separation however depends on the type of the mixture, and some of the physical properties of its components.
We have 4 types of mixtures:
- Solid/Solid mixtures
- Solid/liquid mixtures
- Liquid/liquid mixtures
- Gas/gas mixtures
Separating Solid/Solid Mixtures
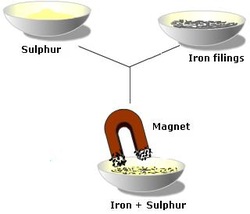
By Magnet:
This method is used to separate a mixture of two solids. One condition must be present though. This is that one of the solids is magnetic. For example if we have a mixture of sand and iron chips. We can separate them by:
This method is used to separate a mixture of two solids. One condition must be present though. This is that one of the solids is magnetic. For example if we have a mixture of sand and iron chips. We can separate them by:
- Pouring the mixture in a dish,
- Introducing a magnet just above the mixture.
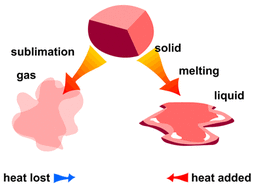
By Sublimation:
If we have a mixture of two solids, one of them undergoes sublimation we can easily separate them by heating the mixture using a Bunsen burner.
One solid might melt while the other one will directly sublime into a gas. This process must be done in a fume cupboard in order to collect the gas.
If we have a mixture of two solids, one of them undergoes sublimation we can easily separate them by heating the mixture using a Bunsen burner.
One solid might melt while the other one will directly sublime into a gas. This process must be done in a fume cupboard in order to collect the gas.
By Solvent Extraction Method:
This method is used one of the solids is water soluble, while the other is insoluble, for example a sand and salt mixture. In this method, the mixture is put in a beaker and water is added to it. The mixture is stirred on gentle heating to make the salt dissolve in the water quickly. Then the mixture is filtered using a filter funnel and filter paper. The residue will be the insoluble sand and the filtrate will be the salt solution. The sand is dried and collected. The salt is obtained from the solution by either the evaporation or the crystallisation method which will be studied later on.
This method is used one of the solids is water soluble, while the other is insoluble, for example a sand and salt mixture. In this method, the mixture is put in a beaker and water is added to it. The mixture is stirred on gentle heating to make the salt dissolve in the water quickly. Then the mixture is filtered using a filter funnel and filter paper. The residue will be the insoluble sand and the filtrate will be the salt solution. The sand is dried and collected. The salt is obtained from the solution by either the evaporation or the crystallisation method which will be studied later on.
Separating Solid/Liquid Mixtures
Solubility: A solution is formed when a solute is dissolved in a solvent.
If you leave a hot saturated solution to cool, crystals of the solute will form. This is because as the temperature decreases the solvent can hold less solute so excess will form in the form of crystals.
The rate of dissolving can be increased by:
If we want to find the solubility of table salt (sodium chloride) at 30oC, we do the follow these steps:
By Evaporation (For Soluble Solid/Liquid Solutions):
By Crystallization (For Soluble Solid/Liquid Solutions):
By Simple Distillation (For Soluble Solid/Liquid Solutions):
- Solute: This is a substance that dissolves in a solvent forming a solution
- Solvent: This is a substance in which a solute dissolves forming a solution
- Solution: A uniform mixture which is formed when a solute is dissolved in a solvent.
- Dilute Solution: A solution with a small amount of solute/dm3.
- Concentrated Solution: A solution with large amounts of solute/dm3.
- Concentration: The amount of solute (in grams or moles) that can dissolve in 1dm3 of a solvent.
- Saturated Solution: A very concentrated solution with the maximum amount of solute that dissolves in it already dissolved in it.
If you leave a hot saturated solution to cool, crystals of the solute will form. This is because as the temperature decreases the solvent can hold less solute so excess will form in the form of crystals.
The rate of dissolving can be increased by:
- Increasing temperature,
- More stirring,
- Crushed solute (larger surface area).
If we want to find the solubility of table salt (sodium chloride) at 30oC, we do the follow these steps:
- Use a balance to measure 100g of water accurately,
- Pour the 100g of water into a beaker,
- Heat the water to 30ºC using a Bunsen burner and a thermometer,
- Using a spatula, add a considerable mass of the table salt into the water and stir,
- If the mass of salt dissolves completely, add the same amount again and stir, repeat this if the mass keeps dissolving completely until you start seeing excess of the salt not dissolving at the bottom of the beaker,
- You have to record the masses of salt you are adding each time and when you start seeing the excess stop adding salt and sum up the amount of salt you added. Call this Mass1,
- Filter the solution. The excess of salt will be the residue, dry it and weigh it. Call this Mass2,
- The amount of table salt that was dissolved in water is Mass1 - Mass2,
- This is the solubility of table salt at 30ºC.
By Evaporation (For Soluble Solid/Liquid Solutions):
- Put solution in a beaker,
- Set the apparatus (Tripod with a gauze above it and a Bunsen burner below it),
- Put the beaker on the gauze,
- Start heating the solution slowly.
By Crystallization (For Soluble Solid/Liquid Solutions):
- Put solution in a beaker,
- Set the apparatus (Tripod with a gauze above it and a Bunsen burner below it),
- Insert a glass rod in the beaker,
- Turn on the Bunsen burner and continuously dip the glass rod in the solution,
- When you see crystals of the solute starting to form on the glass rod, turn of
the Bunsen burner. (This is crystallization point), - Leave the solution to cool,
- Filter the solution and take the crystals, which will be the residue,
- Wash the crystals with distilled water then dry them between two filter papers.
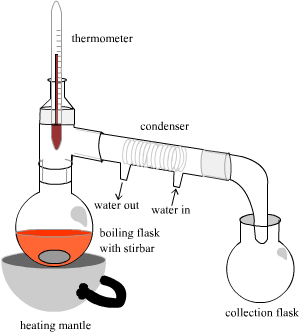
By Simple Distillation (For Soluble Solid/Liquid Solutions):
- Set the apparatus as shown in the diagram below,
- Turn on the Bunsen burner,
- The solvent will evaporate and rise as vapor into the condenser,
- The cold water surrounding the tube where the water is in the condenser will make the vapor condense into liquid,
- The solvent is collected in the tube or beaker on the other side of the condenser, it’s called the distillate,
- The solute is collected in the flask as powder,
- The thermometer must be where the vapor passes the measure the boiling point of the solvent
Filtration (For Insoluble Solid/Liquid Mixtures):
Centrifugation (For Insoluble Solid/Liquid Mixtures):
- Set the apparatus as shown below,
- Pour the mixture into the filter funnel,
- The solvent will go through and be collected in the beaker as the filtrate,
- The insoluble solid will be collected from the funnel as the residue.
Centrifugation (For Insoluble Solid/Liquid Mixtures):
- Put the mixture in a test tube,
- Place the test tube in the centrifugation machine,
- Start the machine.
Separating Liquid/Liquid Mixtures
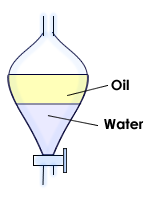 Separating Funnel
Separating Funnel
Separating Funnel (For Immiscible Liquids):
- Immiscible liquids do not mix together; like oil and water.
- If they are put in one container, the denser liquid will settle at the bottom and the lighter one will go above it.
- To separate and oil and water mixture, we pour the mixture into the separating funnel.
- The water is denser than oil, it settles below it.
- The tap is opened to let the water flow into the beaker.
- The tap is closed when all the water is poured, the beaker is replaced by and empty one and the oil is now poured.