The Nature of Matter
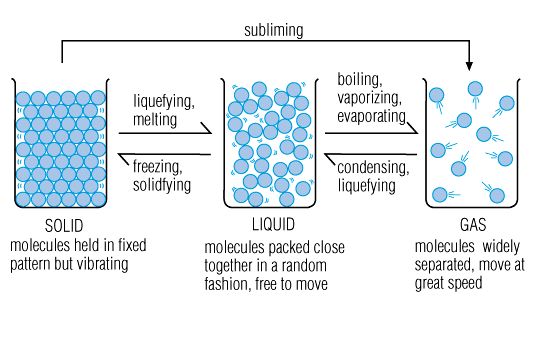
Matter is anything that has mass and occupies space. There are 3 states of matter, solids, liquids and gases.
Solids:
The particles are packed closely together. The forces between particles are strong enough so that the particles cannot move freely but can only vibrate. As a result, a solid has a stable, definite shape, and a definite volume. Solids can only change their shape by force, as when broken or cut.
In crystalline solids, the particles (atoms, molecules, or ions) are packed in a regularly ordered, repeating pattern. There are many different crystal structures, and the same substance can have more than one structure (or solid phase). Solids can be transformed into liquids by melting, and liquids can be transformed into solids by freezing. Solids can also change directly into gases through the process of sublimation.
Liquids:
A liquid is a nearly incompressible fluid which is able to conform to the shape of its container but retains a (nearly) constant volume independent of pressure.
The volume is definite if the temperature and pressure are constant. When a solid is heated above its melting point, it becomes liquid.
This means that the shape of a liquid is not definite but is determined by its container, the most well known exception being water, H2O.
Gases:
A gas is a compressible fluid. Not only will a gas conform to the shape of its container but it will also expand to fill the container.
In a gas, the molecules have enough kinetic energy so that the effect of forces is small, and the typical distance between neighboring molecules is much greater than the molecular size.
A gas has no definite shape or volume, but occupies the entire container in which it is confined. A liquid may be converted to a gas by heating to the boiling point.
The particles are packed closely together. The forces between particles are strong enough so that the particles cannot move freely but can only vibrate. As a result, a solid has a stable, definite shape, and a definite volume. Solids can only change their shape by force, as when broken or cut.
In crystalline solids, the particles (atoms, molecules, or ions) are packed in a regularly ordered, repeating pattern. There are many different crystal structures, and the same substance can have more than one structure (or solid phase). Solids can be transformed into liquids by melting, and liquids can be transformed into solids by freezing. Solids can also change directly into gases through the process of sublimation.
Liquids:
A liquid is a nearly incompressible fluid which is able to conform to the shape of its container but retains a (nearly) constant volume independent of pressure.
The volume is definite if the temperature and pressure are constant. When a solid is heated above its melting point, it becomes liquid.
This means that the shape of a liquid is not definite but is determined by its container, the most well known exception being water, H2O.
Gases:
A gas is a compressible fluid. Not only will a gas conform to the shape of its container but it will also expand to fill the container.
In a gas, the molecules have enough kinetic energy so that the effect of forces is small, and the typical distance between neighboring molecules is much greater than the molecular size.
A gas has no definite shape or volume, but occupies the entire container in which it is confined. A liquid may be converted to a gas by heating to the boiling point.
The Kinetic Theory of Matter States:
The kinetic theory is a theory put together by the finest chemists and physicians of all time. It consists of a number of true facts related to matter and their states. The theory explains the behavior of matter and their physical properties.
The kinetic theory of matter states:
The kinetic theory is a theory put together by the finest chemists and physicians of all time. It consists of a number of true facts related to matter and their states. The theory explains the behavior of matter and their physical properties.
The kinetic theory of matter states:
- All matter is made up of tiny, microscopic moving particles. And each matter has a different type of particles with different size and mass.
- Particles are in continuous movement. All particles are moving all the time in random directions (Brownian motion).
- The speed of movement depends on the mass of the particle, temperature and several other factors that you will know later on.
Melting: The change of state from solid to liquid. The temperature at which a solid melts is called the melting point.
Evaporation: The change of state from liquid to gas. The temperature at which a liquid evaporates is called the boiling point.
Some substances never exist in a liquid form. If they are solid and you heat them they turn into a gas, and if they are a gas and you cool them they turn into a solid. This process is called Sublimation.
The change in state occurs when the temperature is raised or dropped. Melting occurs when you heat a solid because heating gives the particles more kinetic energy making them move faster and further apart, making the solid expand. Until at some point they have enough energy to break the forces of attraction between them and the lattice turning into liquid. If you keep heating the liquid, particles will gain even more kinetic energy and start moving even faster, pushing each other away. The particles at the surface have the highest amount of energy that they can break the forces of attraction and escape as a gas; this is the start of evaporation. At some point, particles will try to escape so quickly that they form bubbles of gas in the liquid. This is the boiling point at which the pressure of the gas forming above the liquid is the same as atmospheric pressure.
On the other hand, cooling a gas will make its particles lose their kinetic energy and move closer and slower. Eventually the forces of attraction will hold them together forming a liquid (condensation). And if a liquid is cooled, its particles will move closer and slower until the forces of attraction are strong enough to hold them tight together forming a solid (freezing).
During the actual change of the state, the temperature of the matter is constant because any heat energy supplied is used to break the bonds. So if you record the temperature change during heating a solid, the temperature will first rise, then it will remain constant for a while (this is the melting point) and then it will rise again.
Evaporation: The change of state from liquid to gas. The temperature at which a liquid evaporates is called the boiling point.
Some substances never exist in a liquid form. If they are solid and you heat them they turn into a gas, and if they are a gas and you cool them they turn into a solid. This process is called Sublimation.
The change in state occurs when the temperature is raised or dropped. Melting occurs when you heat a solid because heating gives the particles more kinetic energy making them move faster and further apart, making the solid expand. Until at some point they have enough energy to break the forces of attraction between them and the lattice turning into liquid. If you keep heating the liquid, particles will gain even more kinetic energy and start moving even faster, pushing each other away. The particles at the surface have the highest amount of energy that they can break the forces of attraction and escape as a gas; this is the start of evaporation. At some point, particles will try to escape so quickly that they form bubbles of gas in the liquid. This is the boiling point at which the pressure of the gas forming above the liquid is the same as atmospheric pressure.
On the other hand, cooling a gas will make its particles lose their kinetic energy and move closer and slower. Eventually the forces of attraction will hold them together forming a liquid (condensation). And if a liquid is cooled, its particles will move closer and slower until the forces of attraction are strong enough to hold them tight together forming a solid (freezing).
During the actual change of the state, the temperature of the matter is constant because any heat energy supplied is used to break the bonds. So if you record the temperature change during heating a solid, the temperature will first rise, then it will remain constant for a while (this is the melting point) and then it will rise again.
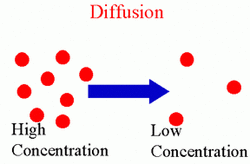
Diffusion:
Diffusion is the random movement of liquid or gas particles to fill the available space and spread evenly. For instance, if you pass by a trash can, you can smell the ugly scent of trash. This is because molecules from the garbage diffused out of the can to the air which you breathed in.
Diffusion rate depends on several factors, these are:
We can also prove diffusion in liquids by a very simple experiment. Pour some water in a beaker, then add a drop of blue ink in the water. After a period of time, you will find that the black ink spread throughout the water and turned it into a blue solution. This was caused by diffusion.
Diffusion is the random movement of liquid or gas particles to fill the available space and spread evenly. For instance, if you pass by a trash can, you can smell the ugly scent of trash. This is because molecules from the garbage diffused out of the can to the air which you breathed in.
Diffusion rate depends on several factors, these are:
- Mass of the substance. The lighter the substance (lower Mr or Ar) the faster it diffuses
- Temperature. The more kinetic energy the particles have, the faster they move and diffuse.
- Presence of other substance. Diffusion is faster when it occurs in an area where there are fewer particles of other substances present. This is why diffusion is extremely fast in vacuums. This is because the diffusing particles have less other particles to stand in their way.
- Intermolecular spaces. This is why gases diffuse faster than liquids and solids do not diffuse.
We can also prove diffusion in liquids by a very simple experiment. Pour some water in a beaker, then add a drop of blue ink in the water. After a period of time, you will find that the black ink spread throughout the water and turned it into a blue solution. This was caused by diffusion.